Promising Lab Results May Kickstart NSCLC Clinical Trials
Study shows a combo of two existing medicines shrink tumors in mice with certain non-small cell lung cancers—clinical trials in humans may follow soon.
The Challenge
When human genome data was shared with the global scientific community, it paved the way for personalized medicine, dramatically improving patient care. For cancers driven by specific genetic mutations, oncology teams can now use that data to predict which treatments will work best for cancer patients and prescribe targeted therapies.
Precision medicine can be highly effective, but its effectiveness is still limited for many types of cancer, including non-small cell lung cancer (NSCLC) with the STK11 gene mutation. That subtype accounts for at least 20% of all lung adenocarcinomas, the most common type of NSCLC.
STK11 is a gene that helps keep tumors from forming and growing (a tumor suppressor gene), and it codes for the protein LKB1, a tumor suppressor protein. But how STK11/LKB1 functions to deter tumors is multifaceted and not completely understood.
When the STK11 gene is mutated, it no longer prevents cells from growing uncontrollably, and the LKB1 protein it codes for is also changed.
Each year, about 50,000 people in the United States are diagnosed with this type of lung cancer. The currently available therapies - including chemotherapy, immunotherapy, and targeted therapy - have been found to have limited effectiveness for people with STK11/LKB1-mutant lung cancer. The overall survival time after diagnosis is less than 1 year. To help save lives, more effective treatments for this type of lung cancer are urgently needed.
The Research
In her lab at Northwestern University Feinberg School of Medicine, Lillian J. Eichner, PhD, recently co-led a collaborative study with the Salk Institute that appeared in Science Advances. That study explored a new targeted therapy option to treat mice that had been genetically engineered to develop NSCLCs with the LKB1 mutation. The study was partially funded through Eichner’s American Cancer Society (ACS) postdoctoral fellowship and set the foundation for two more ACS-funded grants for Eichner.
Our work demonstrates the potential of a new targeted drug therapy using two available and clinically tolerable drugs that effectively shrink lung tumors in mice with NSCLC. Our hope is that starting to test the combination in human clinical trials will feasible within a relatively short timeframe since the two drugs already exist and have been shown to be safe in people."
Lillian J. Eichner, PhD
Northwestern University Feinberg School of Medicine
ACS Research Grantee

Eichner's team used a drug that targets a group of enzymes called HDACS.
The research team turned to an existing treatment that the Food and Drug Administration (FDA) had already approved to treat some blood cancers. That drug targets a group of enzymes called histone deacetylases, or HDACs (pronounced H-DACKS), which are associated with the growth of tumors and cancer metastasis.
Treatment with HDAC inhibitors had not been effective in killing solid tumors, including lung adenocarcinomas, but the study authors point out that the existing drugs were aimed at more than one of the 18 HDAC enzymes. Plus, these scientists note that there hadn’t been enough studies in mice to narrow down specific biological roles for each HDAC enzyme and which of those would be the most effective drug target.
Based on findings from previous studies, Eichner’s lab team focuses on one HDAC: HDAC3, which is the target for existing HDAC inhibitors.
The researchers discovered that lung cancers require HDAC3 to grow and survive.
First, they wanted to learn more about the role of HDAC3 in solid tumors in mice. “We started out believing that a subgroup of HDAC enzymes was part of the cause of LKB1-mutant lung cancer, but we were surprised to discover that lung cancers require HDAC3 enzymes to grow and survive,” Eichner says.
Next, the researchers wanted to learn whether they could slow or stop tumor growth by using a targeted therapy that blocks HDAC3. They evaluated the effect of two medicines based on previous research—one is FDA-approved and the other is in clinical trials. Eicher’s team tested the two drugs at the same time on mice with LKB1-mutated NCSLC.
They tested two drugs that block HDAC3.
One was the targeted therapy trametinib (brand name Mekinist). It’s approved by the FDA to treat certain types of NSCLC, as well as certain types of melanoma, thyroid cancer, and brain cancer. Past research showed that using trametinib alone to kill tumors caused the NSCLC tumors to reduce in size at first but then stopped shrinking—a sign that the cancer was becoming resistant to that treatment.
The other inhibitor they tested, entinostat, is an HDAC inhibitor that's currently in clinical trials. Entinostat selectively targets a group of HDAC enzymes, including HDAC3, that is key to LKB1-mutated tumors, so Eichner’s team thought it could be used for tumors that developed resistance to trametinib.
For 42 days, the team treated mice with LKB1-mutated lung cancer with entinostat, trametinib, or a combination of the two drugs. When used alone, neither drug slowed the tumor’s growth. But when both drugs were used to treat the mice, the tumors had 79% less volume and the mice had 63% fewer tumors in the lungs than the untreated mice. Their study confirmed that entinostat is a viable treatment option in cases where a tumor becomes resistant to trametinib.
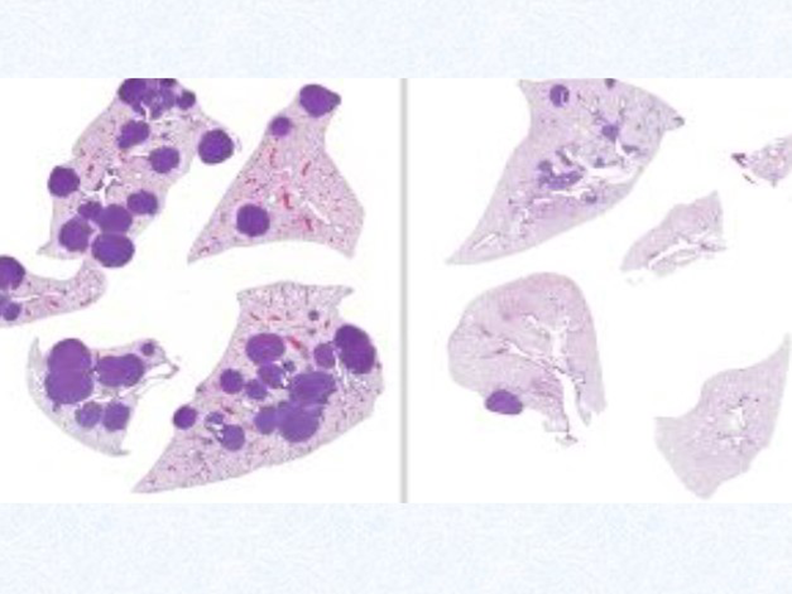
This illustration shows tumors in the lungs of mice with LKB1-mutated non-small cell lung cancer (NSCLC). The dark circles in the left image show tumors from mice that had not received drug treatment. The right image shows reduced numbers and sizes of tumors after treatment with two different types of drugs that are not currently used together for lung cancer. Credit: Salk Institute
Dr. Eichner is an American Cancer Society (ACS) 2023 ResearcHERS Scholar, an honor recognizing trailblazing women scientists for their innovation and potential for high-impact research and for their affiliation with communities and institutions that donate to the ResearcHERS program. In addition to being funded by ACS during her postdoctoral training, she’s recently been awarded grants from ACS that include a Mission Boost grant, which helps fund research that accelerates bench-to-bedside studies, and a Research Scholar Grant.
Why Does it Matter?
“Our findings help to build a knowledge base regarding how to successfully overcome resistance to targeted therapies in people with NSCLCs, as a combination of these drugs shows promise as a potential treatment for people with this hard-to-treat lung cancer,” Eichner says.
Because Eichner and her research team made use of an existing FDA-approved drug and a drug already in clinical trials, getting to the stage where this combo treatment can be used to start helping people with LKB1-mutated lung adenocarcinomas may avoid the lengthy and expensive process of bringing a new drug to market. Their findings have the potential to be moved into human clinical trials and lead to new targeted therapy approaches for treating people with NSCLCs.
The researchers believe this discovery could be transformative for cancers beyond NSCLC as well.


