Your gift is 100% tax deductible
Scientists ID Signals That May Trigger BRCA1 Breast Cancer
An ACS-funded researcher may have found a future drug target to help prevent BRCA1-related breast cancer – signals from the stroma to the epithelium.
The Challenge
About 55% to 72% of women who inherit a damaged (mutated) BRCA1 gene will develop breast cancer by age 70 or 80. That’s a much higher risk compared to women in the general population who don’t have the mutant gene - about 13% of them will develop the disease. Plus, people who have inherited this damaged gene tend to develop cancer at younger ages than people who haven’t, and they’re more likely to develop cancer in both breasts.
These high risks have long kept scientists seeking answers about why and how BRCA1-associated breast cancer develops. They hope that a better understanding of this cancer’s biological causes will lead to better prevention, detection, and treatment.
About 10% of breast cancers develop in lobules and are called invasive lobular carcinomas. Most often, breast cancers develop in the milk ducts and are called invasive ductal carcinomas. The word carcinoma describes tumors that start in the epithelial cells.
Many studies have found that when a damaged BRCA1 gene leads to breast cancer, it starts with changes in the epithelial cells that line the inside of the ducts, called luminal cells. The specific cells where cancer starts are often called luminal progenitor cells.
There have been fewer studies about how other types of breast cells, particularly stromal cells, may change and contribute to the development of cancer, especially as it relates to hereditary genetic mutations such as BRCA1.
Featured Term:
Stroma
The stroma is the cells and tissues that support and give structure to organs, glands, or other tissues in the body. It’s made mostly of connective tissue (made mostly of fibroblasts), blood vessels, lymphatic vessels, immune cells, and nerves.
The stroma provides nutrients to other tissues and organs, removes waste and extra fluid, and may be involved in the body's immune response by modulating inflammation levels. When stromal cells secrete growth factors, they promote tumor growth, invasion, and metastasis.
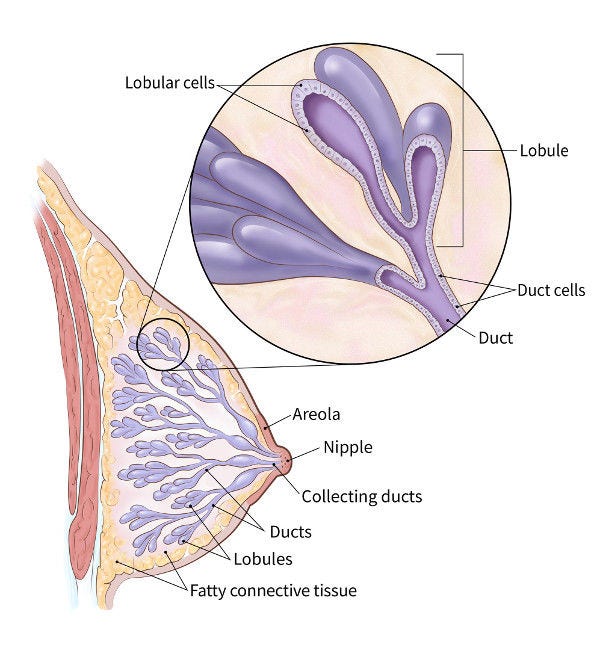
Breasts in women and men are mainly glands, ducts, and fatty tissue. During pregnancy and nursing, the glands (called lobules) make milk, which flows into the milk ducts until it reaches the nipple. The ducts are lined by the epithelium, which is made of two layers of epithelial cells (labeled duct cells above). On the outside are myoepithelial cells and on the inside are luminal epithelial cells. Fatty connective tissue, called the stroma, surrounds the lobules and ducts to keep them in place and functioning normally.
The Research
American Cancer Society (ACS) research scholar, Kai Kessenbrock, PhD, studies how cells in the breast develop early changes that lead to breast cancer, specifically BRCA1-associated breast tumors.
He recently published a study in Nature Genetics that involved mice with an inherited mutated BRCA1 gene. The team also analyzed pre-cancerous tissue with and without BRCA1 mutations in a 3D cellular model in the lab.
The epithelial cell may be the first place hereditary breast cancer starts, but something else—in the stroma, we think—is influencing those epithelial cells to mutate.”
Kai Kessenbrock, PhD
University of California, Irvine
ACS Grantee

“In the past, scientists were primarily focused on BRCA1’s effect on epithelial cells. In our lab, we found that women with germline BRCA1 mutations have distinct precancerous changes within various stromal cells—those cells outside of and around the ducts," Kessenbrock says.
“That means the epithelial cell may be the first place hereditary breast cancer starts, but something else—in the stroma, we think—is influencing those epithelial cells to mutate,” he explains.
Kessenbrock and his team found that breast tissue with a mutated BRCA1 gene but without any cancer present had many more luminal progenitor cells that had changed from their normal state. The cells also had more genes prompting tumor cells to grow rapidly.
The team found two striking differences between human cells in their lab that were BRCA1-mutation carriers and that were noncarriers:
- In BRCA1-mutated carriers, cells that help make up the connective tissue in the stroma - called fibroblasts - had a cancer-associated type (known as a CAF) before any cancer had developed.
- Those BRCA1-mutated fibroblasts had higher amounts of the gene that codes for the enzyme MMP3 (matrix metalloproteinase), which promotes breast cancer during aging and increases genetic instability.
The researchers also revealed something that had been completely unknown: BRCA1-mutated carriers had more MMP3-positive stromal cells close to epithelial structures, suggesting a direct link between MMP3 and an increased risk of developing breast cancer.
Plus, the increase in MMP3 was “particularly significant” around the lobules. The study authors note that this location could indicate that tumors with a BRCA1 mutation may start “predominately in lobular rather than ductal regions.”
They had similar findings in their mouse studies.

These images compare breast cells from mice without cancer (top) with tumors dissected after 6 weeks of growth in mice with normal fibroblasts (middle) and tumors dissected in the same amount of time in mice with MMP3 fibroblasts (bottom). Tumors developed more frequently in the +MMP3 group, demonstrating that fibroblast-derived MMP3 promotes BRCA1-mutated tumors in vivo.
Why Does It Matter?
Kessenbrock’s findings about MMP3 levels in the breast stroma and its location add new evidence to other reports that point to BRCA1-mutated breast cancers starting in luminal progenitor cells. More studies still need to be done, but Kessenbrock is hopeful.
“Our findings that stromal cells cause hereditary breast cancer in mice may help lead to new ways of monitoring and treating people with BRCA1 mutations. What’s more, anti-cancer drugs that block the effects of MMP3 may one day have the potential to prevent these breast cancers in women with high-risk BRCA1 mutations,” he says.
Past studies using MMP3 inhibitor drugs have not shown promising results. But the study authors note that those trials focused on people with late-stage cancers. To learn how well targeting stromal-epithelial interactions might work requires a study designed to include people with a mutated BRCA1 gene who have not developed cancer.
- 21 Metabolites Linked with Breast Cancer (Y Wang)
- AI Tool Improves Breast Cancer Prognosis Accuracy
- Black Women & Genetic Testing (J Palmer)
- Coffee Risks for Colorectal Cancer (C Um)
- Colorectal Cancer Research Highlights
- CPS-3 Disparities Studies
- CPS-3 Researchers Ask What People Eat and Check Urine Samples (Y Wang)



