New Device Aims to Use Light to Predict How Well Chemotherapy Treats Breast Cancer
In his lab at Boston University, Darren Roblyer, PhD, studies mice, invents new medical devices, and guides a clinical trial for women getting chemotherapy as their first treatment for breast cancer. All 3 of these projects are funded by his grant from the American Cancer Society. They all also have the same goal: To learn how well chemotherapy is working to destroy a tumor, as quickly as possible after the treatment is started.
With that information, patients may not have to cope with side effects of chemotherapy drugs that aren’t helping them. Oncologists can try another combination of drugs right away. And, women with breast cancer may survive longer.
Roblyer’s expertise is using light to take pictures of what’s happening inside a tumor. His field of work is called optical imaging.
How Optical Imaging Works
If you’ve ever put a flashlight against your palm, you’ve seen how light shines through skin and spreads out on the other side of your hand. Some health and medical devices make use of these optical traits.
One example is a Fitbit that monitors heart rate by shining a green light through the skin above the wrist. Another optical device is a pulse oximeter. That’s the little clamp nurse’s put on a patient’s finger in the hospital to measure the percentage of blood that’s carrying oxygen. The oximeter works by shining a red light and an infrared light through one side of your finger and using a detector on the other side to measure any light that your finger didn’t absorb.
Roblyer uses near infrared light, which is a little bit redder than humans can see. “What’s cool about light in the near infrared is that it can penetrate deep into tissue—centimeters deep,” Roblyer says. He and his team send light through the skin of the person with cancer, and photons (particles of light) travel through the tissue and through the tumor.
Then, a tiny number of the photons come back out of the tissue. “We can look at the photons with a camera or sensor,” Roblyer says, “and based on how they’ve changed—how many were absorbed, how many scattered—we can tell an amazing amount about the tissue.”
If Digital Optical Spectroscopy (DOS) becomes part of the standard of care, cancer patients could receive only drugs that effectively treat their tumors. And doctors might be able to make timely changes in chemotherapy to avoid side effects for drugs that aren't working.
What Optical Imaging Shows About a Tumor
A common reason someone has chemotherapy as the first treatment for breast cancer is to shrink a tumor so it is easier to remove with surgery. That can mean the difference between having the whole breast removed (mastectomy) and having only the tumor removed (lumpectomy).
To see how effectively chemotherapy is decreasing the tumor’s size, doctors can use traditional imaging devices like mammography and ultrasound.
But in up to about 20% of patients, surgeons find that chemotherapy didn’t shrink the tumor at all. “That means a patient may have had months of treatment that’s making them feel horrible but that’s not doing anything to the cancer,” Roblyer says.
In addition to shrinking the tumor, another big benefit of chemotherapy before surgery can include increasing how long patients live. And that isn’t related to the tumor’s size or shape.
Studies show a woman lives longer when chemotherapy before surgery leads to a “pathologic complete response.” That means when the tumor is removed by surgery, doctors don’t see cancer cells actively growing in the tumor.
“In the infusion center, one major issue for doctors is that you can’t tell if chemo even gets to the tumor,” Roblyer says. His team’s optical imaging methods could change this.
Diffuse Optical Spectroscopy (DOS) lets doctors see the biological changes occurring inside the tumor earlier. The first time Roblyer and his team use DOS is at the same time patients are receiving chemotherapy. They also measure patients during the first week after infusion and at other times during the treatment cycle.
DOS can track a tumor’s blood supply and metabolism by taking images of how the tumor affects light. These kinds of changes occur before changes in tumor shape or size do.
The kinds of imaging currently used to check for a pathologic complete response, include certain types of PET and MRI scans. Because their size makes these machines unmovable, PET scans and MRIs can’t be used during chemotherapy infusions. Doctors tend to use these scans after a patient has received 1 or 2 cycles of chemotherapy.
There are several benefits of seeing how the chemotherapy affects the tumor early on. First, doctors can stop using chemotherapy drugs that aren’t working on the cancer—maybe before they can cause major side effects. Plus, doctors may be able to use DOS to predict how well a different drug or set of drugs will work to help them choose the most promising one(s). Finding the chemotherapy that leads to a pathologic complete response earlier might increase a patient’s chance for surviving longer.
Some Early Exciting Findings
“It’s really, really fascinating,” Roblyer says. Some of the changes his team sees are predictive for how well the chemotherapy will work. “You’d think that you’d see less oxygen in the tumor as it starts to die from chemo,” Roblyer says, “But, we found something kind of weird. In the first 24 hours after a patient’s very first infusion, sometimes we saw an increase in oxygenated blood, not a decrease.”
“Even more provocatively,” he adds, “in earlier studies, we discovered that spike was a predictor of whether the patient would respond to the treatment, weeks and months into the future. It seems to be a positive indicator that the chemo will work well.”
Roblyer and his team were inspired. “We started to think, ‘Wow, there’s probably a lot of biology happening during treatment that hasn’t been studied because the tools haven’t been available to measure patients at the right time points.”
That’s what stimulated his team to design a wearable device that would use optics to study chemotherapy’s effect on a breast tumor. But, as Roblyer says, “You can’t build something and just start measuring patients. There are many steps in between.” And that’s why Roblyer and his group simultaneously test optics in both mice and humans.
The Benefits of Studying Mice and Humans at the Same Time
In text books and journal articles, Roblyer says, it looks like research happens sequentially—a direct path from working with cells, then small animals in a preclinical setting, then with people in clinical trials. “But in our lab, it’s all simultaneous, all the time.” Even his grant covers 3 distinct, but overlapping projects.
“We don’t just translate what we’ve learned from the lab to the clinic like you hear about with drug development,” Roblyer explains. “We translate in both directions—back and forth from the clinic to the lab. I call it reverse translation.”
The benefit, he says, is that you can do things using small animals that you can’t do in humans. For instance, you can try drugs that aren’t FDA approved yet or different doses. “So, we may see something in clinical studies that raises a question we can only answer with an animal study.”
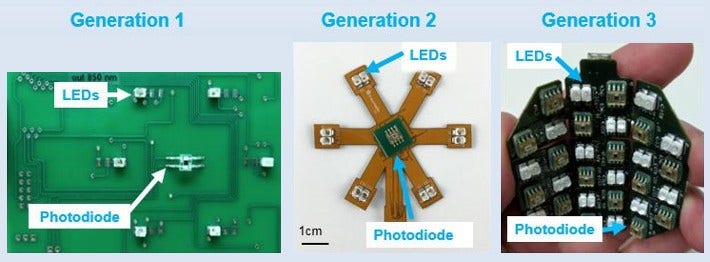
An early design of Robyler’s wearable DOS was rigid and only had 1 light source (LED) and 1 light absorber (photodiode). With generations that followed, he and his team added flexibility to conform with the natural shape of the breast and added more light sources and photodiodes.
Designing a New
Wearable Optical Device
For the clinical trial Roblyer’s co-leading with oncologist Naomi Ko, MD, he uses a handheld probe against the surface of the skin to measure a tumor with DOS. At the same time, he’s designing a flexible DOS that women can wear that conforms to the same shape of their breast.
It will be the first-ever wearable imaging pad that can take continuous optical measurements in breast cancer patients. “It’s been an iterative process.” Roblyer is perfecting his device with the help of a grant from the American Cancer Society.
The current model has 32 light sources (LEDs) and 16 photodiodes. Roblyer's team kept modifying the places for light sources and diodes to find one that would allow the highest sensitivity to measure what they wanted at different depths. Right now, the device doesn’t store data. It has to be connected to a computer to download its information.
“It’s hard to measure as frequently as we want with the device we have,” Roblyer says. “We’re thinking about how to make it untethered in the future because the chemotherapy infusion center is just the first place we want to measure the effects of chemo on the tumor. It would be fantastic if the patient could wear the device at home so we could learn more about what the most valuable timepoints are. They could be a day, or a week after treatment.”
In addition to portability, there are some things to work out with the device—like Roblyer’s team is still looking for the best way to adhere it to the skin. He still has another year of work through his American Cancer Society grant, though, so his team has room for more innovation.
When the grant's over, Roblyer and his team likely have several years of work to do before the wearable DOS device will be ready for doctors to use it with patients. "We still need to test it with healthy volunteers and with people who have breast cancer," Roblyer says. "Plus, FDA approval, and much more."
But simultaneous work continues. Receiving the grant from the American Cancer Society spurred Roblyer’s success with grants from other sources. He and his team are working on several new projects. They’re using optical monitoring to explore less toxic treatments for chemo-resistant breast tumors. They’re also using DOS to monitor the effects of treatment in children with osteosarcoma, a cancer that starts in the bones.
A Phantom in the Optics Lab
A big part of a cancer scientist’s job world is validating that the research they’re doing is accurate and can be used safely in humans. One tool Roblyer’s lab uses to help make sure their measurements are correct is called an optical phantom.
In a research lab, a phantom is a model of human tissue that can be used to test medical devices. Optical phantoms are designed to mimic how light is distributed in human tissue. Roblyer’s team makes optical phantoms with a piece of silicone mixed with a type of dye that absorbs light and particles of titanium dioxide that scatter light.
“We can make distinct types of phantoms. Some absorb light like a tumor, and some absorb light like normal tissue. We can make phantoms that are curved like the human breast or flat like skin,” he says.
Roblyer’s team uses phantoms to:
- simulate how light will be distributed in the
breast with and without tumors - calibrate their DOS to make sure its measuring
accurately - record a measurement for reference
“Imaging phantoms have been used for decades,” he explains. “There are phantoms for X-rays, MRIs, and ultrasound. They’re considered the gold standard for testing a device, and everyone needs standards to test against.”
American Cancer Society news stories are copyrighted material and are not intended to be used as press releases. For reprint requests, please see our Content Usage Policy.




